|
2755
Bristol Street, Suite 285
Costa
Mesa, CA 92626
T
714-549-5837
gcarpenter@cnsresponse.com
|
 |
2755
Bristol Street, Suite 285
Costa
Mesa, CA 92626
T
714-549-5837
gcarpenter@cnsresponse.com
|
|
|
•
|
The
Company’s major multi-site clinical trial (the “Depression Efficacy
trial”) hadn’t completed recruiting and the Company didn’t have the money
to complete it.
|
|
|
•
|
Vendor
payables had been stretched, some for years, and many vendors looked like
they could be moving to termination or
litigation.
|
|
|
•
|
Paid
test volume was static, as was the
database.
|
|
|
•
|
The
Company had never scheduled an Annual Meeting under Len
Brandt.
|
|
|
•
|
CNS
Response was no longer a recognized corporate entity in Delaware, its
state of incorporation, due to failure to pay its corporate franchise
tax.
|
|
1.
|
Complete
the Depression Efficacy Trial
|
|
|
•
|
Situation
in April: The biggest single investment the Company has
made to date is its current multi-site Depression Efficacy
Trial. At the beginning of April, the Company began notifying
sites that it wouldn’t make payments on past invoices, due to a lack of
capital. There was deep concern within the team that sites
would terminate -- or slow -- their work with us, jeopardizing completion
of the trial.
|
|
|
•
|
90-day
objective: We resolved to complete recruiting in all sites by June,
perform an interim validation analysis to confirm that the study is
sufficiently powered, and endeavor to announce study results by
year-end.
|
|
|
•
|
Actions
& Results: Full effort has been maintained at all active trial
sites. We negotiated extended payment agreements with most
sites, and have honored those agreements. Overall, we’ve
reduced payables outstanding to trial sites by 25% and have increased our
speed of payment considerably.
|
|
|
✓
|
The
impact of continuing our study has been good: as all sites have
continued to participate and follow the study protocol, we are currently
on
track for a November release of top-line data at the U.S.
Psychiatric Congress, and a solid publication strategy
thereafter.
|
|
|
✓
|
Validation
study by biostatistician AMAREX was encouraging: our trial was
“powered” at fewer subjects than originally required, allowing us to end
recruiting for the study at 114 subjects on June 12,
2009.
|
|
2.
|
“Monetize
the test”
|
|
|
•
|
Situation
in April: During
this period, physicians using referenced-EEG®
in their practices continued to report exceptional results for their
patients, and many new physicians began training in the use of rEEG®. So
the question is, can we grow rEEG testing profitably? The
clinical trial is likely to attract significant attention to our
technology, but the real proof for investors will be in our ability to
monetize the test. Fortunately, there are some good
companies who have successfully commercialized similar biomarkers (e.g.
Genomic Health - GHDX), and we’re following their well-worn
path.
|
|
|
•
|
90-day
objective: Get the service team and physician network focused on an
achievable goal -- 100 tests per month by 12/09 -- with a
limited commercialization budget.
|
|
|
•
|
Actions
& Results
|
|
✓
|
Listening
to our physician partners has produced immediate
results. There’s always the question with new medical
technologies as to how widely they will be accepted and adopted by
physicians. rEEG is no different. However, there are
a dozen “early adopters” of rEEG who overcame those obstacles, and we can
learn much from them. For the first time, these users now
collaborate on a regular basis with the company and each other, sharing
best practices and product recommendations. We’ve invested
ourselves not only in improving the rEEG test, but in improving the
marketing and logistics of that test inside each physician
practice. And
we’re finally
moving forward with critical product development
projects to enhance our report and database.
|
|||
|
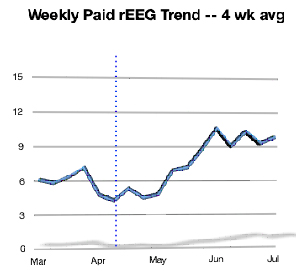
Test volume is up
sharply, mostly as a result of focus and attention on the
basics of testing operations with these top
physicians. Nevertheless, the numbers are still small and will
require a sustained effort to achieve our December goal.
|
 |
|||
|
|
✓
|
Applying
the marketing
lessons from our clinical trial: long after the study
was “powered” and recruiting goals were achieved, a relatively modest
investment in web and other media was still generating
referrals. We are beginning to apply many of the same web-based
tools to generate testing volume within our physician
network.
|
|
|
✓
|
Management
review: we have organized our team to maximize focus and
accomplishment of short-term goals. As part of this
process, an independent management consultant interviewed each member of
the team shortly after the transition. Their report identified
significant improvement in productivity and morale since
April. Shareholders are welcome to contact any of our employees
(or ex-employees) to assess this improvement in management effectiveness
for themselves.
|
|
3.
|
Raise $4-5 million in
capital
|
|
|
·
|
Situation: Under
Len Brandt’s leadership, the Company spent considerable time and money
during 2008 and early 2009 attempting to raise money, but failed to
conclude any transaction for reasons which remain
unclear. The Company’s inability to raise money put the
company squarely in the position where it could only do a dilutive
financing once its cash reserves had been depleted. This
occurred in April.
|
|
|
•
|
Objective:
As of April we estimated that it would require $2.0 million to
operate the Company and complete the trial through the end of
2009, $480,000 to retire overdue payables to clinical trial
sites, and $600,000 to retire debt. Since April, we have
incurred unanticipated legal and other costs related to Len
Brandt’s actions which we estimate will exceed $300,000 by year
end. So, to run the company through year-end required a
minimum of $3,380,000, leading us to conclude that an equity financing
from $4-5 million would be necessary for the company to implement its
plan. We have raised $1.2 million of that amount so far in
convertible bridge notes, as outlined
below.
|
|
|
•
|
Actions
& Results:
|
|
|
✓
|
We
secured a well-known, successful venture capitalist -- John Pappajohn --
as lead investor. John brought our story and business
plans to institutions who will be critical to future equity
financing. Additionally, John invested $1 million in
bridge loans to the company, and SAIL Ventures invested another
$200,000. These funds were immediately employed to execute the
operating objectives described
above.
|
|
|
✓
|
The
company retired $150,000 in overdue vendor payables and $125,000 in
company debt. The Company is fully paid up and in good
standing in Delaware.
|
|
|
✓
|
The
practical effect of this effort has been to stabilize the company, take 5
million shares off the capitalization table, and position
the company to raise equity at a better
valuation than would have been possible
before.
|